Osaka Metropolitan University Rewriting Future: New Molecules Reversibly Change with Light and Heat
Scientists investigating photoswitching molecules, which change their properties when irradiated, have been finding possible applications for these materials, ranging from photopharmacology to storage.
This is a Press Release edited by StorageNewsletter.com on October 22, 2024 at 2:01 pmIn this age of cloud storage, few people are backing up data on CD-RWs. The technology to rewrite data on compact discs was made possible by phase-change materials altered by the light and heat of lasers, though this had a limit of 1,000 rewrites.
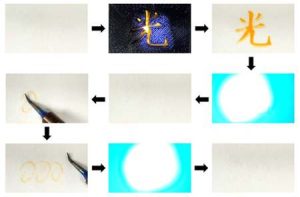
Beginning to see the light UV light writes the Chinese character for ‘light’ on treated paper,
which then gets erased after irradiation with visible light.
A soldering iron then makes marks on treated paper that are erased with visible light irradiation.
(Credit: Osaka Metropolitan University)
Today, scientists investigating photoswitching molecules, which change their properties when irradiated, have been finding possible applications for these materials, ranging from photopharmacology to storage.
Shota Hamatani, student, Graduate School of Engineering, Osaka Metropolitan University, Dr. Daichi Kitagawa, lecturer, and professor Seiya Kobatake, synthesized aza-diarylethenes, which have nitrogen in place of carbon in a molecular structure similar to known photoswitching diarylethenes. The new aza-diarylethenes exhibited not only photoswitching, but thermal switching as well.
They demonstrated that the new photoswitching molecules can be used as a rewritable recording medium, using light or heat to write, and erasing with light.
“Our findings are very useful for the development of switching molecules that can be reversibly altered not only by light, but also by heat,” Kitagawa proclaimed. “They may also lead to the development of new functional materials.”
The findings were published in Angewandte Chemie International Edition (see below).
Funding:
This work was partly supported by JSPS KAKENHI Grant Numbers JP22J21941 (S.H.), JP21KK0092, JP23K26619, JP24K01458 (D.K.), and JP21H02016 (S.K.), and Iketani Science and Technology Foundation (D.K).
Article: Aza-Diarylethenes Undergoing Both Photochemically and Thermally Reversible Electrocyclic Reactions
Angewandte Chemie International Edition has published an article written by Shota Hamatani, Osaka Metropolitan University, Department of Chemistry and Bioengineering, Graduate School of Engineering, Japan, Daichi Kitagawa, Osaka Metropolitan University, Department of Chemistry and Bioengineering Graduate School of Engineering, Sugimoto 3-3-138, Sumiyoshi-ku, 558-8585 Osaka, Japa, and Seiya Kobatake, Osaka Metropolitan University, Department of Chemistry and Bioengineering, Graduate School of Engineering, Japan.
Abstract: “Exploring novel molecular photoswitches plays a crucial role in the field of photo-functional materials chemistry. In this study, we synthesized aza-diarylethenes with benzothiophene-S,S-dioxide as a part of hexatriene structure and investigated their photochromic properties. Unlike previously reported aza-diarylethenes, which exhibit fast thermally reversible photochromism, the compounds synthesized here exhibited pseudo-photochemically reversible photochromism. Due to their thermal stability, we successfully isolated the colored isomer. X-ray crystallographic analysis revealed for the first time that the colored isomer adopts a closed-ring structure with a bond between carbon and nitrogen atoms. Remarkably, these aza-diarylethenes exhibited not only photochemical ring-closing and ring-opening reactions but also thermal ring-closing and ring-opening reactions, driven by a thermal equilibrium between the open- and closed-ring isomers. This behavior, unprecedented for common diarylethenes, was elucidated through kinetic analysis, revealing an energy-level diagram for the thermal equilibrium between these isomers. Furthermore, 1H NMR spectroscopy revealed that both photochemically and thermally generated closed-ring isomers adopt the same structure, which was well explained based on the reaction mechanism of photochemical and thermal ring-closing reactions. These findings not only advance the field of aza-diarylethenes but also inspire future research in the development of new photoswitches.“














 Subscribe to our free daily newsletter
Subscribe to our free daily newsletter


